Designing Empathetic Virtual Clinical Trials, by Leemor Yuravlivker
Design thinking can be applied to any problem to create a solution iteratively, impactfully, and innovatively. And the key to success of clinical trials in the future will be designing them to meet patients where they are.
Pain points of clinical trials
Leemore is at MSK and has worked extensively with clinical trials. For someone who is going through a clinical trial, there are four steps: awareness, onboarding, participation, and monitoring. But there are pain points.
Pain points:
- Screening is rigorous and time consuming
- Consent forms have medical jargon, they’re overwhelming
- Filling out a log is stressful and a pain. It’s not convenient.
- Traveling to the hospital is very inconvenient.
A single clinical trials site might need to use dozens of systems. It’s very confusing. Covid then threw a wrench into things even more so.
Case study: How to adapt and improve the clinical trial experience
This case study happened during Covid, which was a great opportunity to rethink the clinical trial experience. The catch: how to improve the experience without compromising the trial’s integrity?
They used design thinking, making sure to solve the right problems for the right people. Their framework of choice was: Discover, define, design, and deliver. (the Double Diamond approach)
Discover: how do patients experience clinical trials today? Lots of research, hearing pain points from hundreds of patients.
Define: what problem should we focus on solving? The team looked specifically for ways to decrease the face to face moments. They decided to focus on the pill diary experience. It’s often done on paper and handed in to the care team at the hospital.
Design: what does the new experience look like?
Deliver: produce it.
Trends in empathetic clinical trials
Big picture goal: meeting patients where they are.
Current trends:
- Involve patients early in trial design
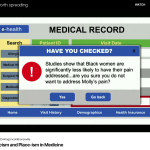
- Closing the equity gap
What would this mean for a patient in future empathetic clinical trials?
- Do screenings from home
- Interactive consent form, written in plain language
- Medication shipped to patient’s home
- Digitized data collection and remote monitoring
- Expanded reality appointments (not always in person)
All of this means a patient can spend more time doing what they love. And then they spend less time with us, in a clinical trial.